
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Reviews
- Pathophysiology
- Epicardial Adipose Tissue and Heart Failure, Friend or Foe?
- Dong-Hyuk Cho, Seong-Mi Park
- Received June 20, 2023 Accepted December 11, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0190 [Epub ahead of print]
- 933 View
- 75 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Heart failure (HF) management guidelines recommend individualized assessments based on HF phenotypes. Adiposity is a known risk factor for HF. Recently, there has been an increased interest in organ-specific adiposity, specifically the role of the epicardial adipose tissue (EAT), in HF risk. EAT is easily assessable through various imaging modalities and is anatomically and functionally connected to the myocardium. In pathological conditions, EAT secretes inflammatory cytokines, releases excessive fatty acids, and increases mechanical load on the myocardium, resulting in myocardial remodeling. EAT plays a pathophysiological role in characterizing both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). In HFrEF, EAT volume is reduced, reflecting an impaired metabolic reservoir, whereas in HFpEF, the amount of EAT is associated with worse biomarker and hemodynamic profiles, indicating increased EAT activity. Studies have examined the possibility of therapeutically targeting EAT, and recent studies using sodium glucose cotransporter 2 inhibitors have shown potential in reducing EAT volume. However, further research is required to determine the clinical implications of reducing EAT activity in patients with HF.
-
Citations
Citations to this article as recorded by- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review
Jorge E. Jalil, Luigi Gabrielli, María Paz Ocaranza, Paul MacNab, Rodrigo Fernández, Bruno Grassi, Paulina Jofré, Hugo Verdejo, Monica Acevedo, Samuel Cordova, Luis Sanhueza, Douglas Greig
International Journal of Molecular Sciences.2024; 25(8): 4407. CrossRef
- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review
- Guideline/Fact Sheet
- Evaluation and Management of Patients with Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
- Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon, The Committee of Clinical Practice Guidelines, Korean Diabetes Association and Committee of Clinical Practice Guidelines, Korean Society of Heart Failure
- Diabetes Metab J. 2023;47(1):10-26. Published online January 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0420
- 4,405 View
- 413 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
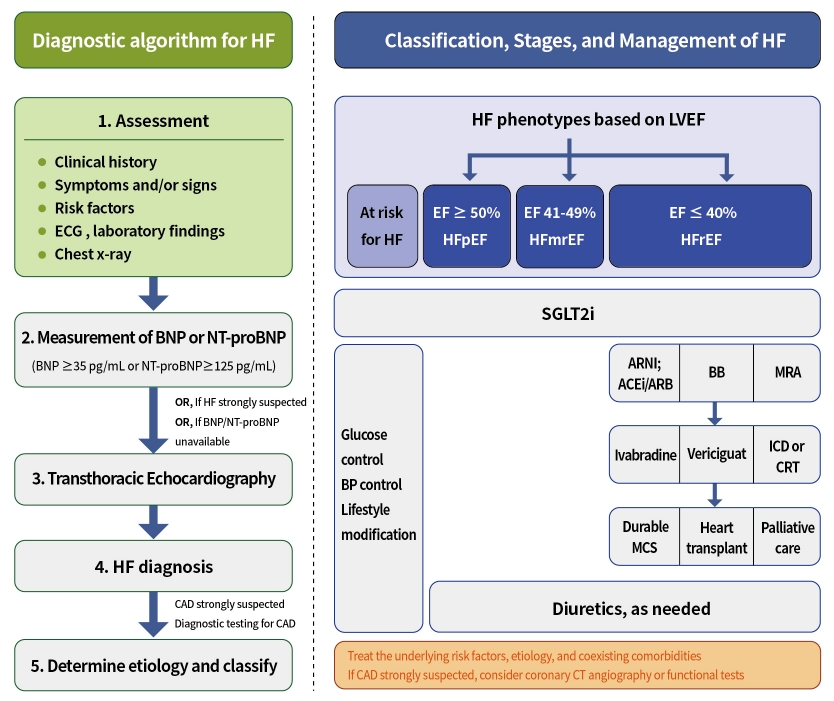
ePub - Diabetes mellitus is a major risk factor for the development of heart failure. Furthermore, the prognosis of heart failure is worse in patients with diabetes mellitus than in those without it. Therefore, early diagnosis and proper management of heart failure in patients with diabetes mellitus are important. This review discusses the current criteria for diagnosis and screening tools for heart failure and the currently recommended pharmacological therapies for heart failure. We also highlight the effects of anti-diabetic medications on heart failure.
-
Citations
Citations to this article as recorded by- A Multicenter, Randomized, Open-Label Study to Compare the Effects of Gemigliptin Add-on or Escalation of Metformin Dose on Glycemic Control and Safety in Patients with Inadequately Controlled Type 2 Diabetes Mellitus Treated with Metformin and SGLT-2 Inh
Hae Jin Kim, Jung Hyun Noh, Min Kyong Moon, Sung Hee Choi, Seung-Hyun Ko, Eun-Jung Rhee, Kyu Yeon Hur, In-Kyung Jeong, Mark Yorek
Journal of Diabetes Research.2024; 2024: 1. CrossRef - Comparison of the effects of gemigliptin versus glimepiride on cardiac function in patients with type 2 diabetes uncontrolled with metformin: The gemi‐heart study
Seung Min Chung, Jun Sung Moon, Jun Hwa Hong, In‐Chang Hwang, Soo Lim
Diabetes, Obesity and Metabolism.2023; 25(8): 2181. CrossRef - Optimization of guideline-directed medical treatment for heart failure patients with reduced ejection fraction
Minjung Bak, Jin-Oh Choi
The Korean Journal of Internal Medicine.2023; 38(5): 595. CrossRef
- A Multicenter, Randomized, Open-Label Study to Compare the Effects of Gemigliptin Add-on or Escalation of Metformin Dose on Glycemic Control and Safety in Patients with Inadequately Controlled Type 2 Diabetes Mellitus Treated with Metformin and SGLT-2 Inh
Original Article
- Metabolic Risk/Epidemiology
- Longitudinal Change in Myocardial Function and Clinical Parameters in Middle-Aged Subjects: A 3-Year Follow-up Study
- Dong-Hyuk Cho, Hyung Joon Joo, Mi-Na Kim, Hee-Dong Kim, Do-Sun Lim, Seong-Mi Park
- Diabetes Metab J. 2021;45(5):719-729. Published online June 15, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0132
- 4,273 View
- 108 Download
- 2 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- Background
Metabolic syndrome (MetS) is closely associated with the aging process. However, changes in metabolic conditions and cardiac function that occur in middle aged population remain unclear. We evaluated longitudinal changes in metabolic parameters and cardiac function during a 3-year period in subjects with suspected MetS.
Methods
We studied 191 participants with suspected MetS at baseline and after 3 years. Anthropometric parameters, including waist circumference (WC), and metabolic parameters, including fasting blood glucose and lipid profile were measured. Conventional echocardiography with two-dimensional speckle tracking was performed.
Results
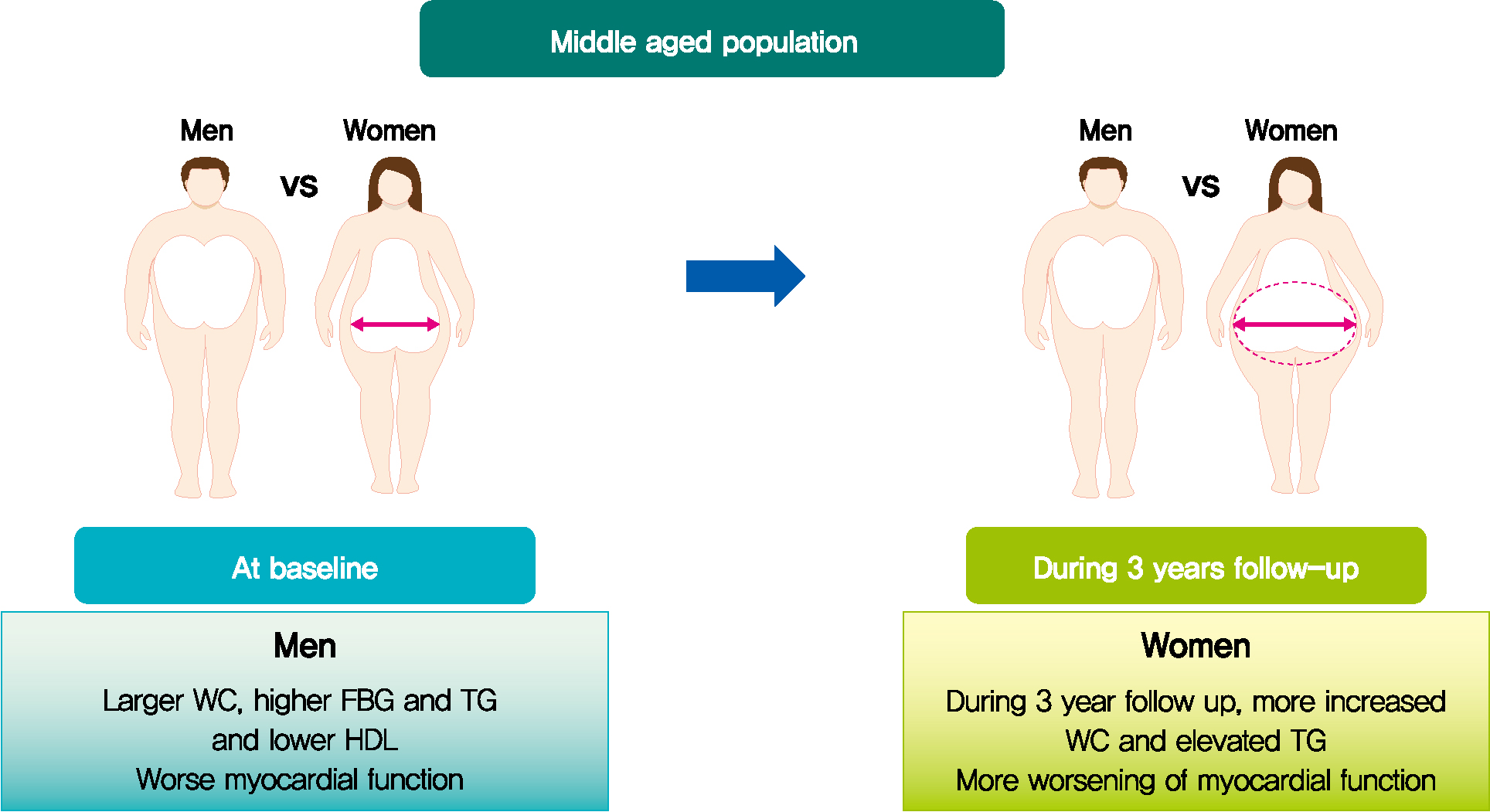
Mean age was 56.2±4.4 years, and there were 97 women (50.8%). Men had increased WC and triglycerides (TG) (WC 91.2±6.8 cm vs. 84.0±8.0 cm, P<0.001; TG 184.4±116.3 mg/dL vs. 128.2±53.6 mg/dL, P<0.001), and reduced global longitudinal strain (GLS) (–15.4%±2.1% vs. –17.1%±2.0%, P<0.001) compared to women. After 3.4 years, values of WC and TG did not change in men but increased in women (all P<0.05). The absolute value of left ventricular (LV) GLS did not change in men but was reduced in women (P=0.011). Change in TG was independently associated with worsening of LV GLS only in women (standardized β, –0.309; 95% confidence interval, –0.130 to –0.009; P=0.025).
Conclusion
In middle aged population, a vulnerable period for metabolic disturbance, cardiac remodeling tended to progress, which was prominent in women. Progression of adiposity and dyslipidemia after menopause may accelerate subclinical cardiac remodeling in middle-aged women. Lifestyle modification and medical interventions may help prevent further cardiac dysfunction in these subjects. -
Citations
Citations to this article as recorded by- Positive additive interaction effects of age, sex, obesity, and metabolic syndrome on left ventricular dysfunction
Dan Zhou, Zhongwen Ye, Zhiqiang Nie, Chaolei Chen, Songyuan Luo, Mengqi Yan, Jiabin Wang, Yingqing Feng
Journal of Diabetes.2024;[Epub] CrossRef - Lung-Heart Outcomes and Mortality through the 2020 COVID-19 Pandemic in a Prospective Cohort of Breast Cancer Radiotherapy Patients
Vincent Vinh-Hung, Olena Gorobets, Nele Adriaenssens, Hilde Van Parijs, Guy Storme, Dirk Verellen, Nam P. Nguyen, Nicolas Magne, Mark De Ridder
Cancers.2022; 14(24): 6241. CrossRef
- Positive additive interaction effects of age, sex, obesity, and metabolic syndrome on left ventricular dysfunction

 KDA
KDA
 First
First Prev
Prev





